Welcome
Coca-Cola under the microscope: “To drink, or not to drink”
Back in 2006 Turkey, for the first time in the world, started a trial against Coca-Cola for the composition of the drink.
Coca-Cola’s label usually says it contains sugar, phosphorous acid, caffeine, caramel, carbon dioxide and some kind of “extract”.
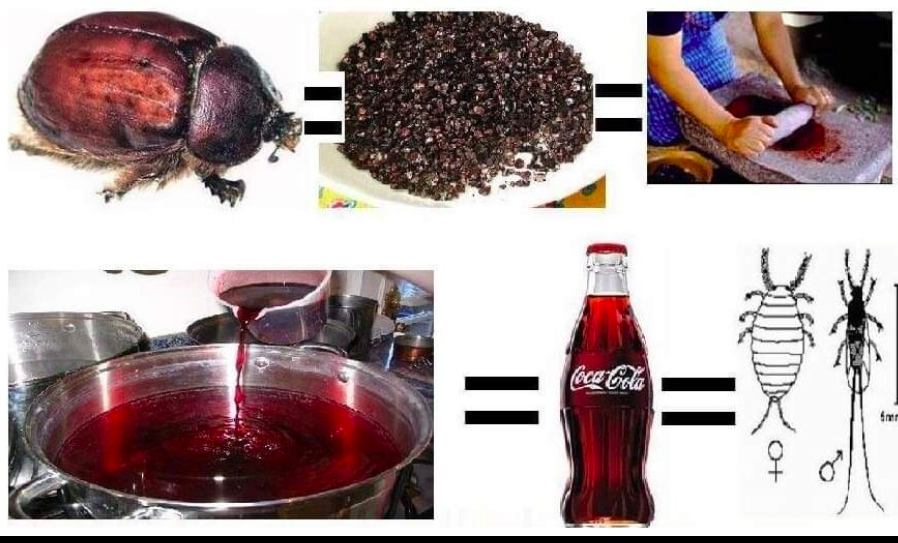
It was this extract that raised suspicion. The Coca-Cola company was forced to reveal the secret of what Coca-Cala is actually made of. It turned out to be a liquid derived from the Cochinil insect.
Cochinil is an insect that lives in the Canary Islands and Mexico. This insect attaches itself to the plant with a hobot, sucks out its juice and never moves from its place. Special fields are being prepared for cochinill insects. These insects in the field are collected by local villagers. From the females and eggs of these insects produce a pigment called lipstick, which colors coca-cola brown. Dried cochinile looks like a raisin, but it’s actually an insect.
You now understand what the word “Coca” means in the name of the drink.
Now let’s talk about what’s behind the word “Cola”
We will tell you the story of an employee who spent 23 years working in a Coca-Cola plant.
Sweet licorice root or “female licorice” (Glycyrrhiza glabra), is used as raw material for the car, with these roots feeding various mammals, including mice. The big companies in the car industry collect these roots by tons by using excavators. At the reunion, they are unable to remove the mice.
Therefore the roots are pressed along with everything contained in them. Only after the pressing are the remnants of fur, paws and whatever else not removed from the root mass. Because the drink has a dark shade, it is not noticed that it also contains blood and bile fluid from mice. Of course, the big car companies try to neutralize the harmful substances with chemicals. For 23 years, this officer telling the story has not had a single cup of coke.
Further into it, even more frightening.
Washington scientists have broken down one of the ingredients of coca-cola into components. It turned out that the caramel contained was not melted sugar at all, but a chemical mixture of sugar, ammonia and sulfites obtained at high pressure and temperature. This “caramel” can cause lung, liver, thyroid and leukemia cancer.
It also became clear that soda involves alcohol – this is the basis of the same secret supplement “7X”.
A few drops of aromatic oils, coriander and cinnamon were added to the alcohol.
The liquid derived from the insect cochinil, called lipstick, has never passed certification, so in some countries the car is not manufactured at all.
How does Coca-Cola’s body react?
After 10 minutes
10 teaspoons of sugar “hit” your system (this is the daily recommended norm).
You won’t start vomiting because phosphorous acid suppresses the action of sugar.
20 minutes from now
There is a jump in insulin levels in the blood.
The liver turns all sugar into fat.
40 minutes from now
Caffeine intake is complete. Your pupils are expanding. Blood pressure rises because the liver releases more sugar in the blood.
Adenosine receptors block, preventing drowsiness.
45 minutes to go
Your body increases the production of the dopamine hormone, which stimulates the brain’s pleasure center.
Heroin has the same principle of action.
One hour later and it’s time to go
Phosphorous acid is linked to calcium, magnesium, and zinc in the intestines, speeding up metabolism. The release of calcium in the urine is increasing.
Just over an hour later
Diuretics are coming into play.
Calcium, magnesium and zinc found in your bones are removed from the body, as well as sodium, electrolytes and water.
Over an hour and a half
You become irritable or lethargic. All the water contained in coca-cola is excreted through the urine.
Coca-Cola’s active ingredient is orthophosphorous acid. His pH is 2.8.
When transporting coca-cola concentrate, the vehicle must be equipped with special containers designed for high corrosion materials.
Here is the detailed composition of the widely advertised Coca-Cola Light product: Sparkling Water, E150d, E952, E951, E338, E330, Aromas, E211.
1. Sparkling water
The presence of carbon dioxide in water stimulates bile secretion, increases the acidity of the stomach and causes meteorism – abundant release of gases. In addition, spring water is not used, but water from the plumbing that passes through special filters.
2. E952 ( Cyclamic Acid. Na, K, calcium salts) – Cyclamic acid and its sodium, potassium and calcium salts
Substitute of sugar. Cyclamat is a synthetic chemical, has a sweet taste 200 times sweeter than sugar and is used as an artificial sweetener. It was banned for use in food products for human consumption because it is carcinogenic, t. well. it causes cancer.
Back in 1969 The Federal Food and Drug Agency (FDA) has banned the cyclama for use in the United States. It has been proven that, like sugar and aspartame, cause bladder cancer in rats. The same year, it was banned for use in Canada. Back in 1975 is banned in Japan, South Korea and Singapore. Prohibited to be used in beverage production in Indonesia. Back in 1979 The World Health Organization is rehabilitating cyclamas, recognizing them harmless. * Safe dose: 0.8 grams per day
3. E150d (Caramel IV – treated with ammonium sulphate dye) Upper sugar obtained by processing sugar at certain temperatures, with or without chemical agents. In this case, ammonium sulfate is added.
4. E950 (potassium acesulfam, acesulfam sodium)
200 times sweeter than sugar. It contains methyl ether, which impairs cardiovascular system function and asparagus acid, which exerts an exhilarating effect on the nervous system and can eventually become addictive. Acesulfam is poorly soluble. Products with this sweetener are not recommended for children, pregnant and lactating mothers. * Safe dose: 1 gram per day
5. E951 (Aspartame) A sweetener for diabetics. Chemically unsustainable – when temperature rises, it decomposes to methanol and phenylalanin. Methanol (metal alcohol) is extremely dangerous – 5 to 10 ml can cause optic nerve death and irreversible blindness, and 30 ml can cause death. In a hot carbonated environment, aspartame turns into formaldehyde, which is an extremely powerful carcinogen.
Cases of aspartame poisoning have been documented, and the symptoms are: loss of sensitivity, headache, fatigue, howling, nausea, heartbeat, weight gain, irritability, anxiety, memory loss, blurred vision or vision loss, rash, seizures, joint pain, depression, spasms, reproductive organ disease, hearing loss. Aspartame may also trigger the following diseases: brain tumor, multiple sclerosis, epilepsy, Based disease, chronic fatigue, Alzheimer’s and Parkinson’s, diabetes, mental retardation, and tuberculosis. * Safe dose: 3 grams a day
6. E338 (orthophosphorous acid, chemical formula – РО3RO4) Fiery and explosive hazard. It irritates the eyes and skin. Application: production of ammonia, sodium, calcium, magnesium, and aluminum, as well as organic synthesis, in activated charcoal and film production, for the production of fireproof materials, fireproof binders, ceramics, glass, fertilizers, synthetic detergents, in medicine, metal cleaning and polishing industry, in the textile industry – in the production of impregnated fireproof fabrics, as well as in the oil and match industries. Nutritional orthophosphorous acid is used in the production of carbonated water and salts (cookie powders). Prevents the absorption of calcium and iron in the body, which can lead to bone tissue loss, osteoporosis. Other side reactions: thirst, skin rash.
7. E330 (lemonic acid) – colorless crystals
Widespread in the wild. Citric acid is obtained from tobacco by fermentation of carbohydrates (sugar, molasses). Applicable to pharmaceutical and food industries. Citric acid salts (citrates) are used in the food industry as acids, preservatives, stabilizers, and in medicine for blood preservation.
8. Fragrances – it is not clear what these fragrance additives are.
9. E211 (sodium benzoate)
Refreshing agent, preservative for food products. Benzoic Acid (E210), sodium benzoate (E211) and potassium benzoat (E212) are added to certain foods as bactericidal and antifungal agents. These are jams, fruit juices, marinades and fruit yoghurt. The use of asthmatic people and people sensitive to aspirin is not recommended. A recent study conducted by professor of molecular biology and biotechnology at the University of Sheffield (England) Peter Piper has found that this compound causes significant DNA damage. According to Piper, sodium benzoate, as an active ingredient in the preservatives used in most carbonated drinks, does not destroy parts of DNA, but deactivate them. This can lead to cirrhosis of the liver and degenerative diseases, such as Parkinson’s.
